The European Joint Programme on Rare Diseases (EJP RD) brings over 130 institutions (including all 24 ERNs) from 35 countries:
- 26 EU Member States (Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Croatia, Ireland, Italy, Netherlands, Latvia, Lithuania, Luxembourg, Malta, Poland, Portugal, Romania, Spain, Sweden, Slovakia, Slovenia)
- 7 associated (Armenia, Georgia, Israel, Norway, Serbia, Switzerland, Türkiye)
- Uk & Canada
to create a comprehensive, sustainable ecosystem allowing a virtuous circle between research, care and medical innovation.
As recognized by the Council Recommendation 2009/C 151/02, rare diseases (RD) are a prime example of a research area that can strongly profit from coordination on a European and international scale. RD research should be improved to overcome fragmentation, leading to efficacious use of data and resources, faster scientific progress and competitiveness, and most importantly to decrease unnecessary hardship and prolonged suffering of RD patients.
In the specific context of the massive generation, need for reuse and efficient interpretation of data, introduction of omics into care practice and the structuration of RD care centers in European Reference Networks, it appears crucial and timely to maximize the potential of already funded tools and programmes by supporting them further, scaling up, linking, and adapting them to the needs of end-users through implementation tests in real settings. To achieve this goal, the EJP RD has two major objectives:
- To improve the integration, the efficacy, the production and the social impact of research on RD through the development, demonstration and promotion of Europe/world-wide sharing of research and clinical data, materials, processes, knowledge and know-how
- To implement and further develop an efficient model of financial support for all types of research on RD (fundamental, clinical, epidemiological, social, economic, health service) coupled with accelerated exploitation of research results for benefit of patients.
To this end, the EJP RD actions are organized within five major Pillars assisted by the central coordination and transversal activities:
Pillar 0: Coordination, Transversal Activities & Communication
Pillar 1: Fundings and Calls
Pillar 2: Coordinated Access to Data and Services
Pillar 3: Training and Empowerment
Pillar 4: Innovation and Clinical Trials Support
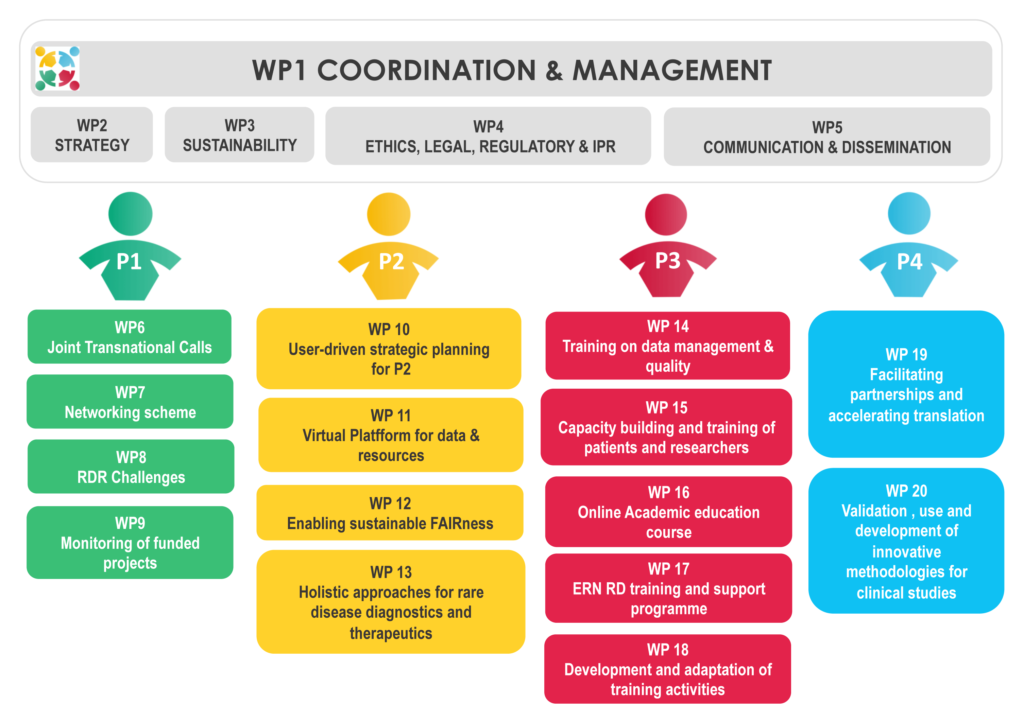

| NUMBER | TITLE | LEAD BENEFICIARY | PILLAR |
|---|---|---|---|
| WP1 | Coordination and management | INSERM | PL0 |
| WP2 | Integrative research and innovation strategy | ISCIII / ISS | PL0 |
| WP3 | Sustainability strategy and business plan | INSERM / EATRIS / ISCIII | PL0 |
| WP4 | Ethical, regulatory, legal and IPR framework of the EJP RD | FGB / FTELE | PL0 |
| WP5 | Communication & dissemination | INSERM | PL0 |
| WP6 | Joint Transnational Calls for collaborative research projects | DLR | PL1 |
| WP7 | Networking to share knowledge on rare diseases | ZonMw | PL1 |
| WP8 | Rare Disease Research Challenges | FFRD | PL1 |
| WP9 | Monitoring of funded projects | CSO-MOH | PL1 |
| WP10 | User-driven strategic planning and transversal activities for PL2 data ecosystem | INSERM | PL2 |
| WP11 | Common virtual platform for discoverable data and resources for RD research | INSERM / CNAG | PL2 |
| WP12 | Enabling sustainable FAIRness and Federation at the record level for RD data, patients and samples | ULEIC / LUMC | PL2 |
| WP13 | Enabling multidisciplinary, holistic approaches for rare disease diagnostics and therapeutics | UM / UKL-HD | PL2 |
| WP14 | Training on data management & quality | ISS | PL3 |
| WP15 | Capacity building and training of patients and researchers in Rare Disease research and processes | EURORDIS | PL3 |
| WP16 | Online Academic education course | FFRD | PL3 |
| WP17 | ERN RD training and support programme | EKUT | PL3 |
| WP18 | Development and adaptation of training activities | VUHSK / IPCZ / EURORDIS | PL3 |
| WP19 | Facilitating partnerships and accelerating translation for higher patient impact | EATRIS / FTELE | PL4 |
| WP20 | Accelerating the validation, use and development of innovative methodologies tailored for clinical trials in RDs | AP-HP / UKA / HSK | PL4 |
| WP21 | Ethics requirements | INSERM | PL0 |

